Expert Answer Step 1 Solution:- Given mass of P A 4 O A 10 = 53.5 g Molar mass of P A 4 O A 10 = 4 ( 31) + 10 ( 16) = 284 g / mol View the full answer Step 2 Step 3 Final answer Previous question Next question Transcribed image text: 53.5 mol of P4O10 contains how many moles of P? moles of P : Not the exact question you’re looking for?
17 31 g of phosphorus P4 and 32 g of oxygen are completely converted into P4O6 and P4O10 respectively,then moles of P4O6 and P4O10 formed respectively will be ??
Explanation: Well clearly, I have 4 ×13 cards in the diamond suit. You propose 68.5⋅ mol of P 4O10, and clearly I have 4 ×68.5 ⋅ mol of phosphorus atoms. And this has a precise numerical value, I,e, 4 × 68.5⋅ mol ×6.022 × 1023 ⋅ mol−1 = 1.65 ⋅ 1026 phosphorus atoms. Answer link

Source Image: scribd.com
Download Image
Best Newest Oldest J.R. S. answered • 02/07/20 Tutor 5.0 (145) Ph.D. University Professor with 10+ years Tutoring Experience About this tutor › 56.0 mol P 4 O 10 x 4 mol P/mol P 4 O 10 = 224 mol P Upvote • 0 Downvote Add comment Report Still looking for help? Get the right answer, fast. Ask a question for free Get a free answer to a quick problem.
Source Image: bartleby.com
Download Image
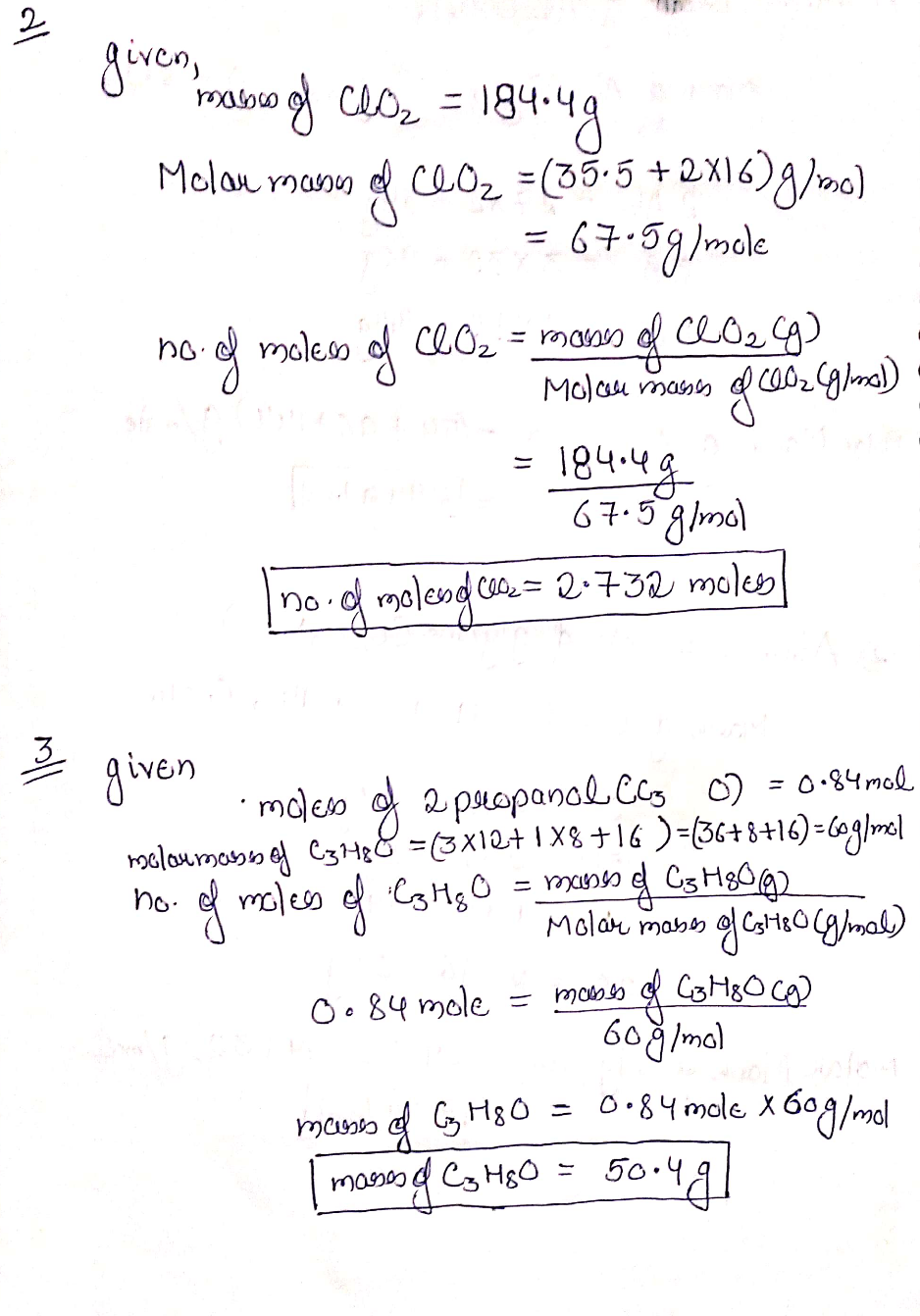
58.5 mol of P4O10 contains how many moles of P? | Homework.Study.com So, to find the number of moles of P in 335 moles of P40 10 we can simply multiply 83.5 moles . by 4 I mole Paolo ki moles of P . 3 3. / 4 moles P4016- 8 moles of p ./ 4 moles of/ Pi= 3 3. 2 mol Paolo x 4 mol P 1 mol Palio. – 132 moles of P. V proves of 23 . 5 moves off Pro10 contains 134 moles of P.

Source Image: slideshare.net
Download Image
33.5 Mol Of P4o10 Contains How Many Moles Of P
So, to find the number of moles of P in 335 moles of P40 10 we can simply multiply 83.5 moles . by 4 I mole Paolo ki moles of P . 3 3. / 4 moles P4016- 8 moles of p ./ 4 moles of/ Pi= 3 3. 2 mol Paolo x 4 mol P 1 mol Palio. – 132 moles of P. V proves of 23 . 5 moves off Pro10 contains 134 moles of P. VIDEO ANSWER: If we have 83.5 moles of the p 410 point, we want to know how many moles of the substance we have. There are 4 phosphorus and 10 oxygen in this formula. … 38.5 mol of P4O10 contains how many moles of P? Instant Video Answer. Instant Text Answer. Step 1/3 1. Write the balanced chemical equation for the reaction of P4O10 with
NWTC General Chemistry Ch 07 | PPT
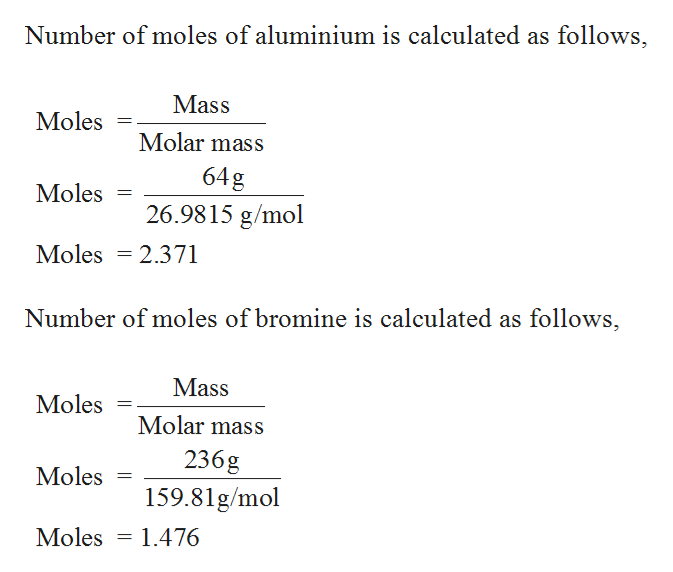
Chemistry Chemistry questions and answers 33.5 mol of P4O10 contains how many of P Question: 33.5 mol of P4O10 contains how many of P 3 3. 5 mol of P 4 O 1 0 contains how many of P This question hasn’t been solved yet! Not what you’re looking for? Submit your question to a subject-matter expert. Send to expert Previous question Next question Answered: If 64.0 g of Al and 236 g of Br2 were… | bartleby
Source Image: bartleby.com
Download Image
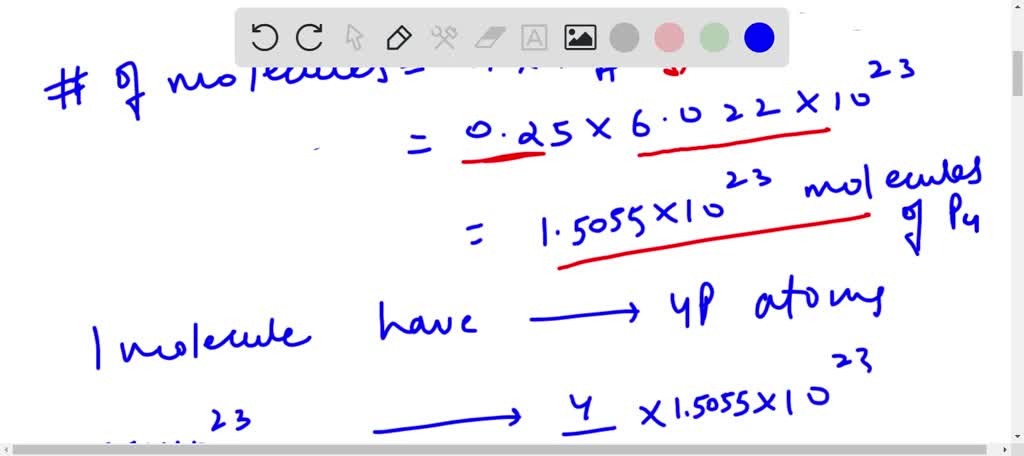
⏩SOLVED:0.25 mol of P4 molecules contains atoms. (a) 1.764 ×10^23… | Numerade Chemistry Chemistry questions and answers 33.5 mol of P4O10 contains how many of P Question: 33.5 mol of P4O10 contains how many of P 3 3. 5 mol of P 4 O 1 0 contains how many of P This question hasn’t been solved yet! Not what you’re looking for? Submit your question to a subject-matter expert. Send to expert Previous question Next question
Source Image: numerade.com
Download Image
17 31 g of phosphorus P4 and 32 g of oxygen are completely converted into P4O6 and P4O10 respectively,then moles of P4O6 and P4O10 formed respectively will be ?? Expert Answer Step 1 Solution:- Given mass of P A 4 O A 10 = 53.5 g Molar mass of P A 4 O A 10 = 4 ( 31) + 10 ( 16) = 284 g / mol View the full answer Step 2 Step 3 Final answer Previous question Next question Transcribed image text: 53.5 mol of P4O10 contains how many moles of P? moles of P : Not the exact question you’re looking for?
Source Image: byjus.com
Download Image
58.5 mol of P4O10 contains how many moles of P? | Homework.Study.com Best Newest Oldest J.R. S. answered • 02/07/20 Tutor 5.0 (145) Ph.D. University Professor with 10+ years Tutoring Experience About this tutor › 56.0 mol P 4 O 10 x 4 mol P/mol P 4 O 10 = 224 mol P Upvote • 0 Downvote Add comment Report Still looking for help? Get the right answer, fast. Ask a question for free Get a free answer to a quick problem.

Source Image: homework.study.com
Download Image
Welcome to Chem Zipper.com……: What are common structural features of oxides (P4O6 and P4O5) of phosphorous? Answer: 33.5 mol of P 4 O 10 contains 134 mol of P. Explanation: 1 mol P 4 O 10 4 mol P. 33.5 molP4O10 × 4 molP 1 molP4O10 = 134 molP.

Source Image: chemzipper.com
Download Image
Answered: A sample of C12H22011 contains 0.4662… | bartleby So, to find the number of moles of P in 335 moles of P40 10 we can simply multiply 83.5 moles . by 4 I mole Paolo ki moles of P . 3 3. / 4 moles P4016- 8 moles of p ./ 4 moles of/ Pi= 3 3. 2 mol Paolo x 4 mol P 1 mol Palio. – 132 moles of P. V proves of 23 . 5 moves off Pro10 contains 134 moles of P.
Source Image: bartleby.com
Download Image
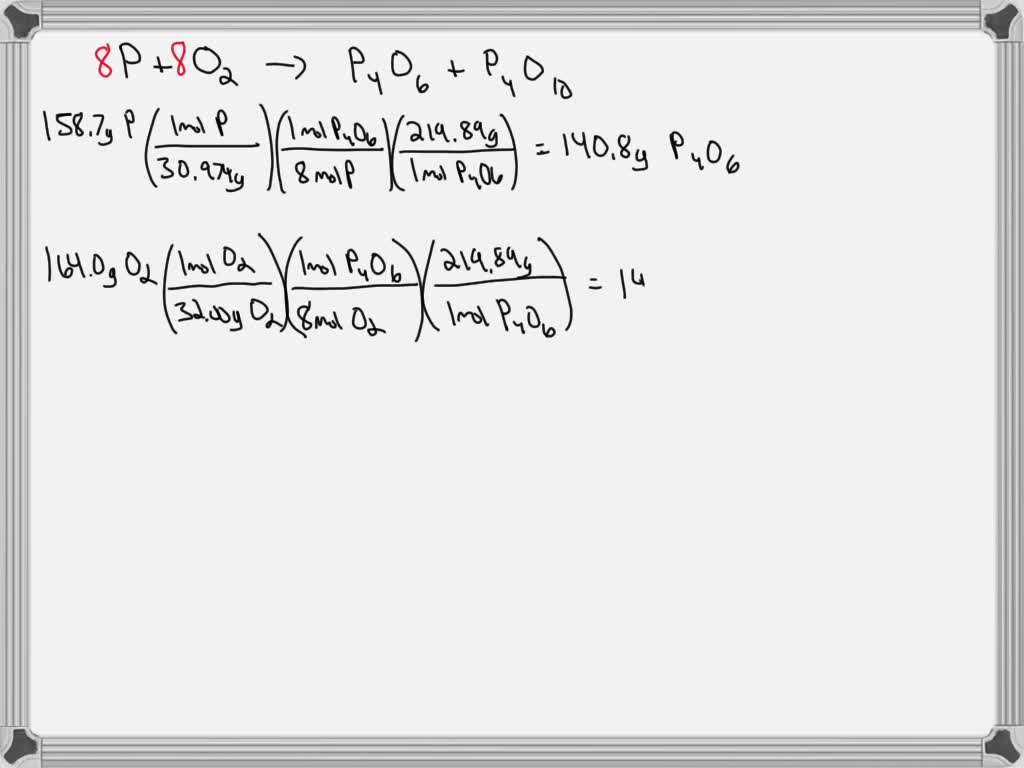
SOLVED: A mixture of 158.7 g of P and 164.0 g of O2 reacts completely to form P4O6 and P4O10. Find the masses of P4O6 and P4O10 that are formed by the VIDEO ANSWER: If we have 83.5 moles of the p 410 point, we want to know how many moles of the substance we have. There are 4 phosphorus and 10 oxygen in this formula. … 38.5 mol of P4O10 contains how many moles of P? Instant Video Answer. Instant Text Answer. Step 1/3 1. Write the balanced chemical equation for the reaction of P4O10 with
Source Image: numerade.com
Download Image
⏩SOLVED:0.25 mol of P4 molecules contains atoms. (a) 1.764 ×10^23… | Numerade
SOLVED: A mixture of 158.7 g of P and 164.0 g of O2 reacts completely to form P4O6 and P4O10. Find the masses of P4O6 and P4O10 that are formed by the Explanation: Well clearly, I have 4 ×13 cards in the diamond suit. You propose 68.5⋅ mol of P 4O10, and clearly I have 4 ×68.5 ⋅ mol of phosphorus atoms. And this has a precise numerical value, I,e, 4 × 68.5⋅ mol ×6.022 × 1023 ⋅ mol−1 = 1.65 ⋅ 1026 phosphorus atoms. Answer link
58.5 mol of P4O10 contains how many moles of P? | Homework.Study.com Answered: A sample of C12H22011 contains 0.4662… | bartleby Answer: 33.5 mol of P 4 O 10 contains 134 mol of P. Explanation: 1 mol P 4 O 10 4 mol P. 33.5 molP4O10 × 4 molP 1 molP4O10 = 134 molP.