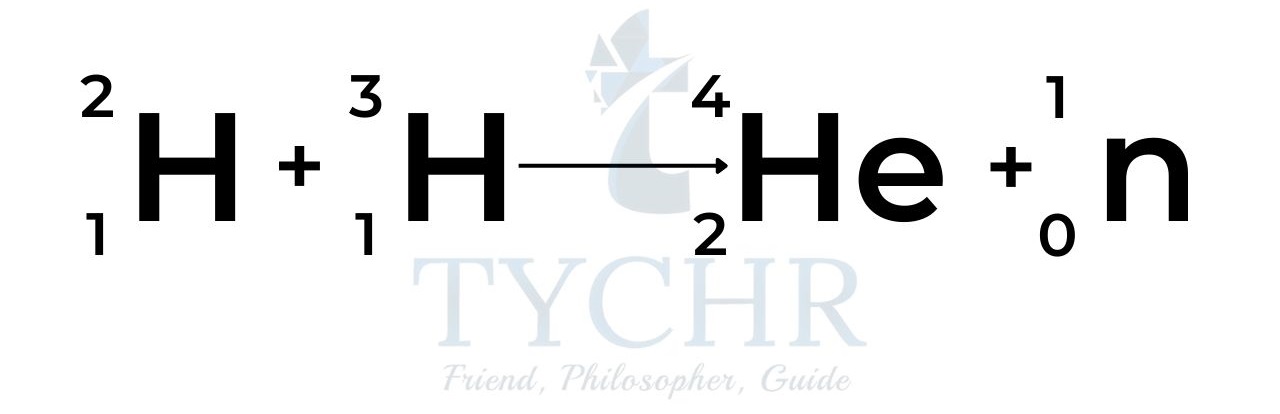
Identify the parent isotope and write a balanced nuclear reaction for each process. Iodine-130 is formed by ejecting an electron and a gamma ray from a nucleus. Uranium-240 is formed by alpha decay. Curium-247 is formed by releasing a helium dication and a gamma ray. Write a balanced nuclear equation for each process.
Exploring Nuclear Energy by NEED Project – Issuu
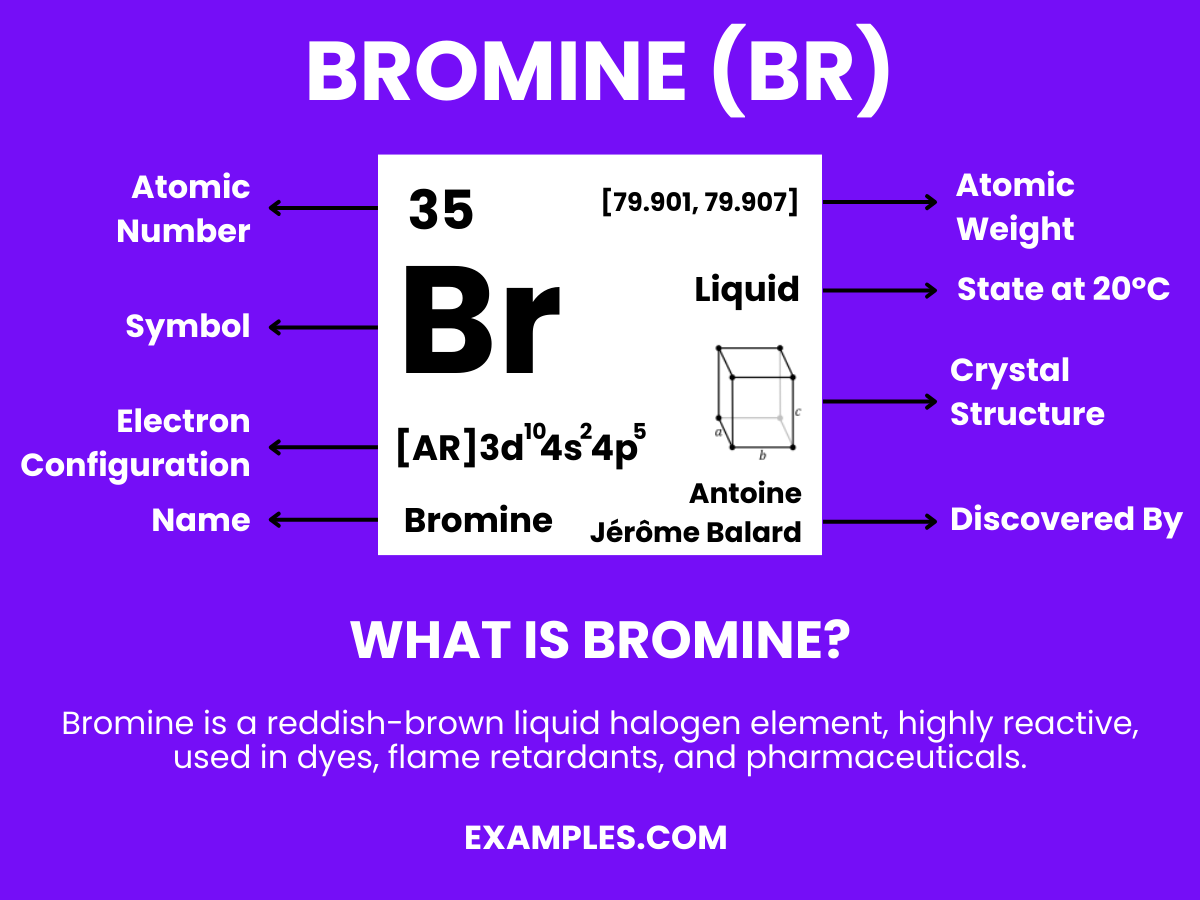
Jun 20, 2022Instant Answer: EXPERT VERIFIED Step 1/2 The atomic number of bromine (Br) is 35, which means it has 35 protons. The number of neutrons is given as 46. The atomic mass number (A) of an atom is the total number of protons and neutrons in its nucleus.
Source Image: tychr.com
Download Image
Oct 8, 202310/08/2023 Chemistry High School verified answered • expert verified Give the nuclear symbol (isotope symbol) for the isotope of bromine, Br, that contains 46 neutrons per atom. a) ^80Br b) ^81Br c) ^82Br d) ^83Br Expert-Verified Answer No one rated this answer yet — why not be the first? 😎 professorenglish94 Final answer:
Source Image: chem4kids.com
Download Image
What is Bromine? – Preparation, Properties, Uses, Compounds, Reactivity Answer The element symbol for oxygen is O and its atomic number is 8. The mass numbers for oxygen must be 8 + 8 = 16; 8 + 9 = 17; 8 + 10 = 18. The nuclear symbols are written this way (again, pretend the superscript and subscript are sitting right on top of each other beside the element symbol): 168 O, 178 O, 188 O Or, you could write:

Source Image: numerade.com
Download Image
Give The Nuclear Symbol For The Isotope Of Bromine
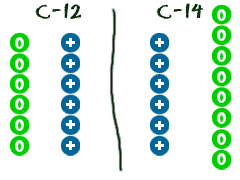
Answer The element symbol for oxygen is O and its atomic number is 8. The mass numbers for oxygen must be 8 + 8 = 16; 8 + 9 = 17; 8 + 10 = 18. The nuclear symbols are written this way (again, pretend the superscript and subscript are sitting right on top of each other beside the element symbol): 168 O, 178 O, 188 O Or, you could write: Bromine (35 Br) has two stable isotopes, 79 Br and 81 Br, and 32 known radioisotopes, the most stable of which is 77 Br, with a half-life of 57.036 hours.. Like the radioactive isotopes of iodine, radioisotopes of bromine, collectively radiobromine, can be used to label biomolecules for nuclear medicine; for example, the positron emitters 75 Br and 76 Br can be used for positron emission
SOLVED: Give the nuclear symbol (isotope symbol) for the isotope of bromine, Br, that contains 46 neutrons per atom. Replace the question marks with the proper integers. nuclear symbol: 80Br
Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope’s mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Additionally, N = A −Z. Example 1: What is the isotopic notation for 35 Br Bromine – Electron Shell Structure | SchoolMyKids | Atomic structure, Element chemistry, Electron configuration

Source Image: pinterest.com
Download Image
What is Boron – Properties of Boron Element – Symbol B | nuclear-power.com Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope’s mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Additionally, N = A −Z. Example 1: What is the isotopic notation for

Source Image: nuclear-power.com
Download Image
Exploring Nuclear Energy by NEED Project – Issuu Identify the parent isotope and write a balanced nuclear reaction for each process. Iodine-130 is formed by ejecting an electron and a gamma ray from a nucleus. Uranium-240 is formed by alpha decay. Curium-247 is formed by releasing a helium dication and a gamma ray. Write a balanced nuclear equation for each process.

Source Image: issuu.com
Download Image
What is Bromine? – Preparation, Properties, Uses, Compounds, Reactivity Oct 8, 202310/08/2023 Chemistry High School verified answered • expert verified Give the nuclear symbol (isotope symbol) for the isotope of bromine, Br, that contains 46 neutrons per atom. a) ^80Br b) ^81Br c) ^82Br d) ^83Br Expert-Verified Answer No one rated this answer yet — why not be the first? 😎 professorenglish94 Final answer:
Source Image: examples.com
Download Image
Solved Give the nuclear symbol (isotope symbol) for the | Chegg.com The Naked Scientists Periodic Table of Videos Created by video journalist Brady Haran working with chemists at The University of Nottingham. Element Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.

Source Image: chegg.com
Download Image
Symbol and electron diagram for bromine Royalty Free Vector Answer The element symbol for oxygen is O and its atomic number is 8. The mass numbers for oxygen must be 8 + 8 = 16; 8 + 9 = 17; 8 + 10 = 18. The nuclear symbols are written this way (again, pretend the superscript and subscript are sitting right on top of each other beside the element symbol): 168 O, 178 O, 188 O Or, you could write:

Source Image: vectorstock.com
Download Image
Elements and Symbols Quiz Study Guide – ppt video online download Bromine (35 Br) has two stable isotopes, 79 Br and 81 Br, and 32 known radioisotopes, the most stable of which is 77 Br, with a half-life of 57.036 hours.. Like the radioactive isotopes of iodine, radioisotopes of bromine, collectively radiobromine, can be used to label biomolecules for nuclear medicine; for example, the positron emitters 75 Br and 76 Br can be used for positron emission

Source Image: slideplayer.com
Download Image
What is Boron – Properties of Boron Element – Symbol B | nuclear-power.com
Elements and Symbols Quiz Study Guide – ppt video online download Jun 20, 2022Instant Answer: EXPERT VERIFIED Step 1/2 The atomic number of bromine (Br) is 35, which means it has 35 protons. The number of neutrons is given as 46. The atomic mass number (A) of an atom is the total number of protons and neutrons in its nucleus.
What is Bromine? – Preparation, Properties, Uses, Compounds, Reactivity Symbol and electron diagram for bromine Royalty Free Vector The Naked Scientists Periodic Table of Videos Created by video journalist Brady Haran working with chemists at The University of Nottingham. Element Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.