Overview. The group 0 elements are placed on the far right of the periodic table. They are called the noble gases because they are gases at room temperature, and they are very unreactive. The noble gases are so unreactive, they are monatomic and exist as separate atoms. The name ‘noble gas’ comes from the idea that the group 0 elements are
Group 18: Noble gases | StudyPug
Jun 30, 2023Group 18: Properties of Nobel Gases. Page ID. The noble gases (Group 18) are located in the far right of the periodic table and were previously referred to as the “inert gases” due to the fact that their filled valence shells (octets) make them extremely nonreactive. The noble gases were characterized relatively late compared to other element
Source Image: sciencewithme.com
Download Image
The noble gas family of elements – helium, neon, argon, krypton, xenon, and radon – had previously been regarded as inert. By combining xenon with a platinum fluoride, Bartlett created the first noble gas compound. This reaction began the field of noble gas chemistry, which became fundamental to the scientific understanding of the chemical bond.

Source Image: oliveboard.in
Download Image
Worksheet noble gases|KS4 Chemistry|Teachit 7 days agoWhen the members of the group were discovered and identified, they were thought to be exceedingly rare, as well as chemically inert, and therefore were called the rare or inert gases. It is now known, however, that several of these elements are quite abundant on Earth and in the rest of the universe, so the designation rare is misleading. Similarly, use of the term inert has the drawback that

Source Image: tes.com
Download Image
Why Are The Noble Gases So Unreactive
7 days agoWhen the members of the group were discovered and identified, they were thought to be exceedingly rare, as well as chemically inert, and therefore were called the rare or inert gases. It is now known, however, that several of these elements are quite abundant on Earth and in the rest of the universe, so the designation rare is misleading. Similarly, use of the term inert has the drawback that The noble gases (historically also the inert gases, sometimes referred to as aerogens) are the naturally occurring members of group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Under standard conditions, these chemical elements are odorless, colorless, monatomic gases with very low chemical reactivity and cryogenic boiling points.
Noble gases – preventing the Hidenberg disaster | Teaching Resources
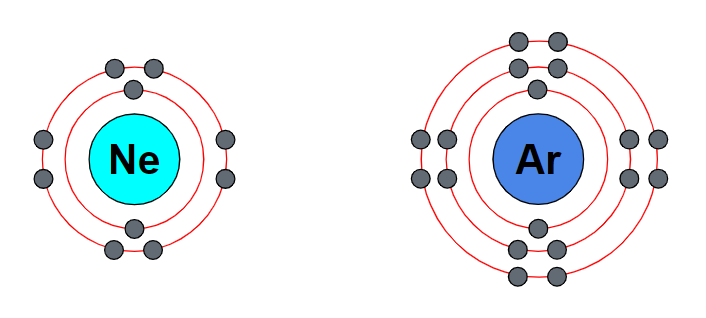
Jul 22, 2023Noble gases are unreactive because they have a completely filled outer electron shell, making them stable. 1 This configuration results in a lack of readily available valence electrons for bonding with other atoms, reducing their tendency to form chemical compounds. Well, this was just a simple answer. The Periodic Table of Elements – Elements in Group 18 – Mr Ruel Tuition
Source Image: mrrueltuition.com
Download Image
KS4 Chemistry Noble Gases. – ppt download Jul 22, 2023Noble gases are unreactive because they have a completely filled outer electron shell, making them stable. 1 This configuration results in a lack of readily available valence electrons for bonding with other atoms, reducing their tendency to form chemical compounds. Well, this was just a simple answer.

Source Image: slideplayer.com
Download Image
Group 18: Noble gases | StudyPug Overview. The group 0 elements are placed on the far right of the periodic table. They are called the noble gases because they are gases at room temperature, and they are very unreactive. The noble gases are so unreactive, they are monatomic and exist as separate atoms. The name ‘noble gas’ comes from the idea that the group 0 elements are

Source Image: studypug.com
Download Image
Worksheet noble gases|KS4 Chemistry|Teachit The noble gas family of elements – helium, neon, argon, krypton, xenon, and radon – had previously been regarded as inert. By combining xenon with a platinum fluoride, Bartlett created the first noble gas compound. This reaction began the field of noble gas chemistry, which became fundamental to the scientific understanding of the chemical bond.
Source Image: teachit.co.uk
Download Image
PPT – Atomic structure PowerPoint Presentation, free download – ID:2050932 Humans Mathematics Chemistry Subscribe now Impossible chemistry: Forcing noble gases to work Noble gases don’t react – or so most of us learned at school. But where there’s a will, there’s

Source Image: slideserve.com
Download Image
Why are noble gases unreactive? – Concepts Berg – PSIBERG 7 days agoWhen the members of the group were discovered and identified, they were thought to be exceedingly rare, as well as chemically inert, and therefore were called the rare or inert gases. It is now known, however, that several of these elements are quite abundant on Earth and in the rest of the universe, so the designation rare is misleading. Similarly, use of the term inert has the drawback that
Source Image: psiberg.com
Download Image
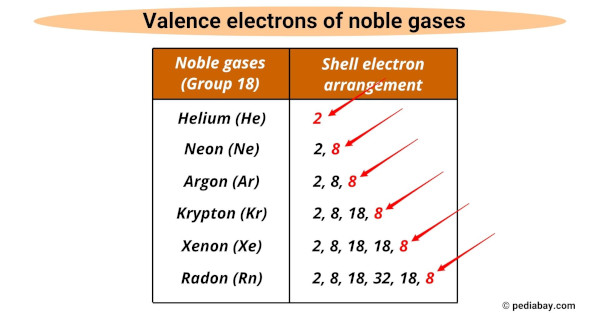
Noble Gases of the Periodic Table – Pediabay The noble gases (historically also the inert gases, sometimes referred to as aerogens) are the naturally occurring members of group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Under standard conditions, these chemical elements are odorless, colorless, monatomic gases with very low chemical reactivity and cryogenic boiling points.
Source Image: pediabay.com
Download Image
KS4 Chemistry Noble Gases. – ppt download
Noble Gases of the Periodic Table – Pediabay Jun 30, 2023Group 18: Properties of Nobel Gases. Page ID. The noble gases (Group 18) are located in the far right of the periodic table and were previously referred to as the “inert gases” due to the fact that their filled valence shells (octets) make them extremely nonreactive. The noble gases were characterized relatively late compared to other element
Worksheet noble gases|KS4 Chemistry|Teachit Why are noble gases unreactive? – Concepts Berg – PSIBERG Humans Mathematics Chemistry Subscribe now Impossible chemistry: Forcing noble gases to work Noble gases don’t react – or so most of us learned at school. But where there’s a will, there’s